A new ‘hybrid’ hydrogel, which allows clinicians to safely deliver stem cells to the site of a brain injury in mice, has been developed by researchers from the University of Melbourne and the Australian National University.
A hydrogel is a water-based gel that can be used to deliver substances into the body and can be used to promote the effective growth of new cells.
Published in Nature Communications, the proof-of-concept breakthrough solves a major challenge faced by stem cell researchers since the 1980s – keeping stem cells alive for long enough to allow them to evolve into the cells required to create new tissue when they are inserted into a damaged part of the body.
The hydrogel supplies both the stem cells and oxygen needed to keep stem cells alive during the injection process and to ensure the stem cells evolve into the type of cells needed to create new tissue to repair damage. Researchers believe this advance will benefit stem cell treatments in many other parts of the body beyond the brain and central nervous system.
The team that developed the hydrogel is co-led by University of Melbourne Professor David Nisbet, Director of The Graeme Clark Institute for Biomedical Engineering; and Australian National University (ANU) Professor Colin Jackson, a member of both the Innovations in Peptide and Protein Science, and Synthetic Biology Australian Research Council Centres of Excellence.
Professor Nisbet said: “After an injury such as a stroke, there is a dead area in the brain, including the blood system. So, we need a temporary blood supply to support cells until the blood system repairs. This patented hydrogel provides that.
“Very few drug treatments can treat conditions like stroke or Parkinson’s Disease and they have little efficacy. There are currently no treatments that can reverse these conditions.”
Professor Jackson said the breakthrough will interest researchers and clinicians globally and is likely to lead to many revolutionary medical treatments.
“Proof of concept has now been demonstrated within the brain of mice, but the research represents a generalisable strategy for developing injectable nanomaterials for a diverse range of applications, including cell transplantation, gene and drug delivery, 3D in vitro disease models and organ-on-a-chip technology,” Professor Jackson said.
Over five years of research, the team discovered that a synthetic protein based on myoglobin – a natural protein found in high concentrations in the heart muscles of sperm whales and horses – added to their hydrogel provided the sustained oxygen release needed to ensure stem cells survive the delivery process and develop into the type of cells needed to repair brain tissue.
Whales and other deep-diving animals are thought to have evolved high concentrations of myoglobin in their muscle tissue so they could slowly absorb as much oxygen as possible while diving. Similarly, horses are thought to have evolved higher concentrations of myoglobin so they could run over longer distances.
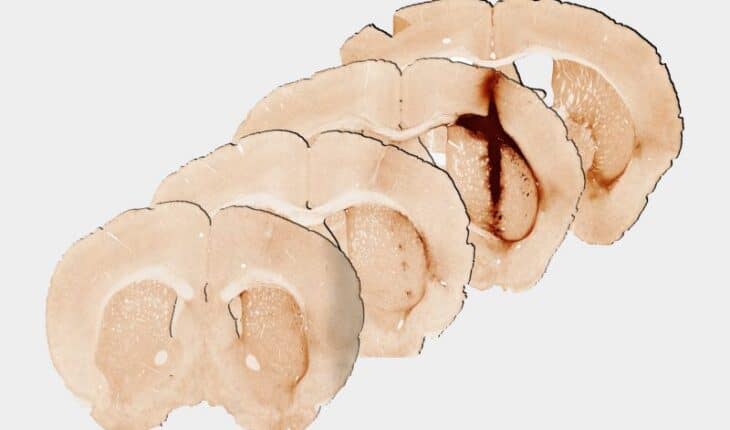
University of Melbourne Professor Clare Parish conducted the mouse studies and said the results were achieved in injured brain tissue, raising the possibility for growing new tissue for future human treatment.
“We saw that the hydrogel incorporating myoglobin and stem cells repaired injured brain tissue. Analysis at 28 days after delivery of the hydrogel revealed significantly enhanced survival and growth of the new stem cells that are needed for healthy brain functioning, compared with a hydrogel without myoglobin,” Professor Parish said.
“We observed that the new tissue could be stimulated in a similar way to healthy brain tissue, providing the first evidence of the benefits of including oxygen delivery within a hydrogel to achieve the long-term survival and integration of stem cell transplants.”
- Gut microbiome could delay onset of type 1 diabetes - 3rd April 2025
- The da Vinci 5 Robot Is Set To Transform Bariatric Care: - 31st March 2025
- Beyond money: the hidden drivers fuelling child food insecurity - 31st March 2025