Pioneering research using bacteria brings scientists a step closer to creating artificial cells with lifelike functionality. Scientists have harnessed the potential of bacteria to help build advanced synthetic cells which mimic real life functionality.
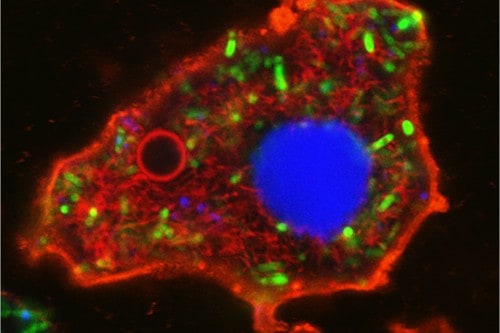
Amoeba-shaped bacteriogenic protocell: membrane (red boundary); nucleus (blue); cytoskeleton (red filaments); vacuole (red circle); ATP production (green). Scale bar, 5 μm. Professor Stephen Mann and Dr Can Xu
The research, led by the University of Bristol and published today in Nature, makes important progress in deploying synthetic cells, known as protocells, to more accurately represent the complex compositions, structure, and function of living cells.
Establishing true-to-life functionality in protocells is a global grand challenge spanning multiple fields, ranging from bottom-up synthetic biology and bioengineering to origin of life research. Previous attempts to model protocells using microcapsules have fallen short, so the team of researchers turned to bacteria to build complex synthetic cells using a living material assembly process.
Professor Stephen Mann from the University of Bristol’s School of Chemistry, and the Max Planck Bristol Centre for Minimal Biologytogether with colleagues Drs Can Xu, Nicolas Martin (currently at the University of Bordeaux) and Mei Li in the Bristol Centre for Protolife Research have demonstrated an approach to the construction of highly complex protocells using viscous micro-droplets filled with living bacteria as a microscopic building site.
In the first step, the team exposed the empty droplets to two types of bacteria. One population spontaneously was captured within the droplets while the other was trapped at the droplet surface.
Then, both types of bacteria were destroyed so that the released cellular components remained trapped inside or on the surface of the droplets to produce membrane-coated bacteriogenic protocells containing thousands of biological molecules, parts and machinery.
The researchers discovered that the protocells were able to produce energy-rich molecules (ATP) via glycolysis and synthesize RNA and proteins by in vitro gene expression, indicating that the inherited bacterial components remained active in the synthetic cells.
Further testing the capacity of this technique, the team employed a series of chemical steps to remodel the bacteriogenic protocells structurally and morphologically. The released bacterial DNA was condensed into a single nucleus-like structure, and the droplet interior infiltrated with a cytoskeletal-like network of protein filaments and membrane-bounded water vacuoles.
As a step towards the construction of a synthetic/living cell entity, the researchers implanted living bacteria into the protocells to generate self-sustainable ATP production and long-term energization for glycolysis, gene expression and cytoskeletal assembly. Curiously, the protoliving constructs adopted an amoeba-like external morphology due to on-site bacterial metabolism and growth to produce a cellular bionic system with integrated life-like properties.
Corresponding author Professor Stephen Mann said: “Achieving high organisational and functional complexity in synthetic cells is difficult especially under close-to-equilibrium conditions. Hopefully, our current bacteriogenic approach will help to increase the complexity of current protocell models, facilitate the integration of myriad biological components and enable the development of energised cytomimetic systems.”
First author Dr Can Xu, Research Associate at the University of Bristol, added: “Our living-material assembly approach provides an opportunity for the bottom-up construction of symbiotic living/synthetic cell constructs. For example, using engineered bacteria it should be possible to fabricate complex modules for development in diagnostic and therapeutic areas of synthetic biology as well as in biomanufacturing and biotechnology in general.”
Paper
- Gut microbiome could delay onset of type 1 diabetes - 3rd April 2025
- The da Vinci 5 Robot Is Set To Transform Bariatric Care: - 31st March 2025
- Beyond money: the hidden drivers fuelling child food insecurity - 31st March 2025