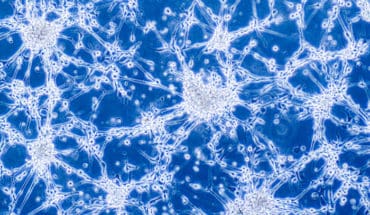
A protein modification of the gene expression regulator MECP2 can convey neuroprotection under inflammation.
Researchers at the University of Eastern Finland have found a potential neuroprotective effect of a protein modification that could be a therapeutic target in early Alzheimer’s disease. The new study investigated the role of MECP2, a regulator of gene expression, in Alzheimer’s disease related processes in brain cells. The study found that phosphorylation of MECP2 protein at a specific amino acid decreases in the brain as Alzheimer’s disease is progressing. Abolishing this phosphorylation of MECP2 in cultured mouse neurons upon inflammatory stimulation enhanced their viability and increased the expression of important genes in the maintenance and protection of neurons. The results obtained in the study call attention to modifications of MECP2 as potential targets for developing specific therapies against AD and other neurodegenerative diseases. The study was recently published in the Cells journal.
Alzheimer’s disease is the most common form of dementia with more than 40 million people living with the disease worldwide. No therapies currently exist for the effective prevention or treatment of the disease. Long before severe symptoms of Alzheimer’s disease, such as memory loss and confusion occur, connections between neurons are lost and neurons are dying in the brain. Neurons are not the only cell type that contributes to Alzheimer’s disease progression. Microglia, the immune cells of the brain, have been shown to contribute to neuronal damage and loss. While mildly activated microglia can protect neurons by removing harmful substances and cell debris, their chronic activation can lead to secretion of neurotoxic factors and engulfment of healthy neurons.
“The function of MECP2 phosphorylation has been almost exclusively studied in neurons. As it has become evident that microglia are involved in the pathogenesis of many neurodegenerative diseases, we chose to focus on microglial cells and co-cultures of neurons with microglia. To additionally model the activation of microglia, we induced inflammation through treatment with different compounds”, says Early Stage Researcher Rebekka Wittrahm.
In post-mortem brain samples from different stages of Alzheimer’s disease, this study found that phosphorylation of the MECP2 protein decreased at the amino acid serine 423 in the early stages of Alzheimer’s disease. Accordingly, the question arose whether MECP2 unphosphorylated at serine 423 would change cellular processes when expressed in cultured microglia or neuron-microglia co-cultures. To study this question, both wild type MECP2 and a variant where serine 423 was mutated to prevent phosphorylation were expressed in the different cell culture models. In microglia cells, the researchers found that overexpression of wildtype MECP2 led to a decrease in phagocytic uptake of particles. Upon activation of the microglia, expression of wildtype MECP2 enhanced their inflammatory response to stimulation. For most of the analyzed parameters, blocked phosphorylation of MECP2 serine 423 did not lead to major changes as compared to the wild type protein.
In contrast to the results from microglial monocultures, the expression of the proteins in neurons co-cultured with microglia cells revealed several differences between wild type and the mutated MECP2. RNA sequencing analysis revealed that blocking of the serine 423 phosphorylation upon microglial activation increased the expression of several neuronal genes that are known to contribute to maintenance and protection of the cells.
“More extensive experiments are needed but it is conceivable that the dephosphorylation of MECP2 at serine 423 might be a mechanism of the cells to mediate neuroprotection in the early stages of Alzheimer’s disease,” Rebekka Wittrahm considers.
In summary, the results of this study suggest that MECP2 serine 423 is an essential phosphorylation site that might play a role in regulating neuronal gene expression upon neuroinflammation. The study calls attention to the idea that modification sites of MECP2 are potential targets for developing specific therapies against Alzheimer’s disease and other neurodegenerative diseases in the future.
- The da Vinci 5 Robot Is Set To Transform Bariatric Care: - 31st March 2025
- Beyond money: the hidden drivers fuelling child food insecurity - 31st March 2025
- Tobacco and Vapes Bill - 31st March 2025